Research
Kinetics and Mechanism of Polymerization Reactions
The study of the kinetics and mechanism of polymerizations has a long tradition at the University of Göttingen. Using pulsed laser methods of many kinds, kinetic coefficients of propagation, termination, transfer and copolymerization in radical polymerization are investigated in our department in the most precise way. Techniques based on the use of lasers to induce polymerization are used to accurately determine rate in homo- and copolymerizations. The data are used for simulations and also to estimate the properties of the polymers formed as a function of the selected reaction conditions. The data are also used to test and extend copolymerization models. Essential methods here are the so-called PLP-SEC method for the determination of kp and time-resolved tracking of the monomer concentration by NIR
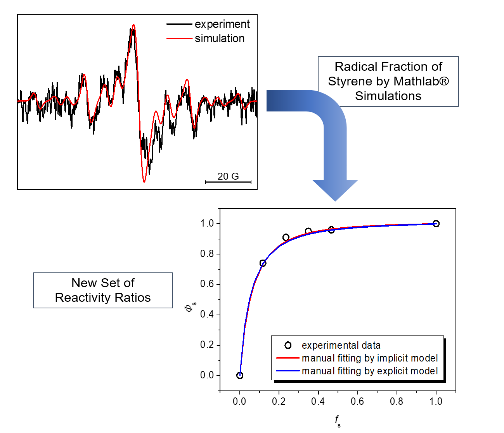
spectroscopy for the determination of kt and other kinetic coefficients. Further detailed insight into the kinetics of radical polymerization is obtained by time-resolved tracking of the radical concentration by ESR spectroscopy. Hereby, the addition and fragmentation steps of the RAFT equilibrium could for instance be accurately measured for the first time. We also investigated the kinetics of surface-bound radical polymerization in terms of propagation and termination rates. We were also able to determine the RAFT equilibrium of surface-bound RAFT polymerizations using time-resolved ESR. In the field of catalytic polymerization, we investigated the kinetics and mechanism of catalyzed chain growth using Zr complexes by time-resolved NMR and computer modeling.